Abstract
Background: The known driver mutations in PMF (including JAK2, CALR, and MPL) do not explain the highly inflammatory phenotype associated with this disease. Moreover, JAK2 inhibitors provide symptomatic relief in PMF, but they are not curative, indicating that our understanding of the molecular pathology of MPNs is incomplete. High-resolution insights into the mutational landscape of MPNs may inform new diagnostic and therapeutic approaches.
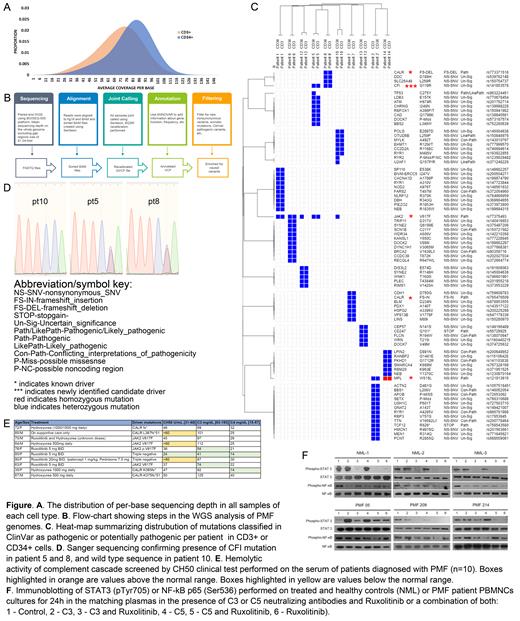
Methods: We performed 60x whole-genome sequencing (WGS) on CD34+ hematopoietic stem/progenitor cells and matched in vitro-expanded CD3+ lymphocytes from 10 patients (pts) with PMF, of whom 5 had JAK2 V617F, 2 had MPL W515L, and 3 had CALR (del52 or ins5) mutations. Paired-end WGS was performed using BGISEQ-500 platform. Clean reads with Q20 and Q30 at 96.4% and 87.1%, respectively, were aligned to the human GRCh37 reference genome. 99.9% reads mapped successfully and 90.6% mapped uniquely. Mean sequencing depth for CD3+ and CD34+ cells were 72x and 83x, respectively (Fig A). Bioinformatic pipeline strategy is summarized on Fig B. We also examined complement activity in sera from 10 PMF pts using CH50 (a screening test for total complement activation) and C3 and C4 component activity. We further evaluated the effect of complement-neutralizing anti-C3 and anti-C5 (C3/C5 NeuAbs) on JAK/STAT and NF-κB signaling in mononuclear cells (MNCs) isolated from peripheral blood of these PMF pts.
Results: ~3.4e6 SNPs were identified (n=10): 99.9% were represented in dbSNP, 98.1% were annotated in GnomAD, and 4,587 were novel. Among ~8.6e5 InDels, 91.9% were represented in dbSNP, 53.8% in GnomAD, and ~6.9e4, were novel. We identified 3,540 copy-number variations (CNVs) and 3,365 SVs. Rare non-synonymous variants (RNSV) were defined as SNVs with a maximum minor allele frequency <0.01 in any GnomAD population. RNSVs were further filtered based on clinical interpretation of their genomic variation in ClinVar. 78 unique RNSVs were identified (Fig C), after exclusion of RNSVs with benign, likely benign, or undetermined status. Known PMF drivers (JAK2 V617F, CALR, and MPL W515L) were present in both CD34+ and CD3+ cells, except in 2 samples JAK2 V617F was present only in CD34+ cells. This suggested WGS-detectable driver clonality in most but not all analyzed cases. All mutations except MPL were heterozygous. We further found known RNSVs in TP53, U2AF1, TCF12, as well as previously unreported in PMF pathogenic mutations in CD247 and OTUD6B. Among 11 RNSVs with conflicting classification in ClinVar, we observed mutations in BRCA2, TTN, APOB, ATM and CDH1 (previously linked to MPNs). Within this group of variants, we also detected a p.G119R mutation in Complement Factor I (CFI, rs141853578) in 2 samples. This RNSV was not previously reported in PMF. CFI G119R was detected in 1 sample with JAK2 and 1 with CALR driver mutation, in both CD34+ and CD3+cells as confirmed by Sanger sequencing (Fig D). CFI encodes a serine proteinase that regulates the complement pathway, and its deficiency is associated with severe inflammatory pathology. In the sera of different cohort of 10 pts with PMF, we observed an increase in CH50 levels (n=3) and depletion of C3 (n=3; Fig E), independent of the presence of specific driver mutations or treatment with ruxolitinib (RUXO) or hydroxyurea, suggesting that complement overactivation may contribute to the pathology of PMF in some pts. We further incubated PMF or healthy PBMCs in matching plasmas in the presence of C3/C5 NeuAbs either alone or in combination with RUXO. In PBMCs from healthy controls, STAT3 pTyr705 decreased upon treatment with RUXO alone or with C3/C5 NeuAbs, and NF-κB pSer536 decreased for all treatment conditions (Fig F). In contrast, in PMF, STAT3 pTyr705 did not consistently decrease in response to RUXO either alone or with C3/C5 NeuAbs. Notably, NF-κB pSer536 was unaffected by RUXO alone, and only treatment with RUXO + C3/C5 NeuAbs induced a decrease in NF-κB pSer536 (Fig F).
Conclusions: Using WGS, we discovered an inactivating RNSV in CFI G119R in 20% of pts with PMF. In a separate cohort of PMF patients we detected elevated serum complement activation. Furthermore, PMF-specific dysregulation of STAT3 and NF-κB activation could be modulated by exposure to C3/C5 NeuAbs. This is a first, to our knowledge, report linking the overactivation of the complement cascade to inflammation-related pathogenesis of PMF.
Hobbs: Novartis: Consultancy; Bayer: Research Funding; Celgene/Bristol Myers Squibb: Consultancy; AbbVie.: Consultancy; Constellation Pharmaceuticals: Consultancy, Research Funding; Incyte Corporation: Research Funding; Merck: Research Funding. Oh: Abbvie: Membership on an entity's Board of Directors or advisory committees; Blueprint Medicines: Membership on an entity's Board of Directors or advisory committees; Celgene Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Constellation: Membership on an entity's Board of Directors or advisory committees; CTI Biopharma: Membership on an entity's Board of Directors or advisory committees; Disc Medicine: Membership on an entity's Board of Directors or advisory committees; Geron: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; Kartos Therapeutics: Membership on an entity's Board of Directors or advisory committees; PharamaEssentia: Membership on an entity's Board of Directors or advisory committees; Sierra Oncology: Membership on an entity's Board of Directors or advisory committees. Di Paola: CSL Behring: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal